In vitro and in situ structural characterization of the biofilm-associated small basic protein (SBP) amyloid
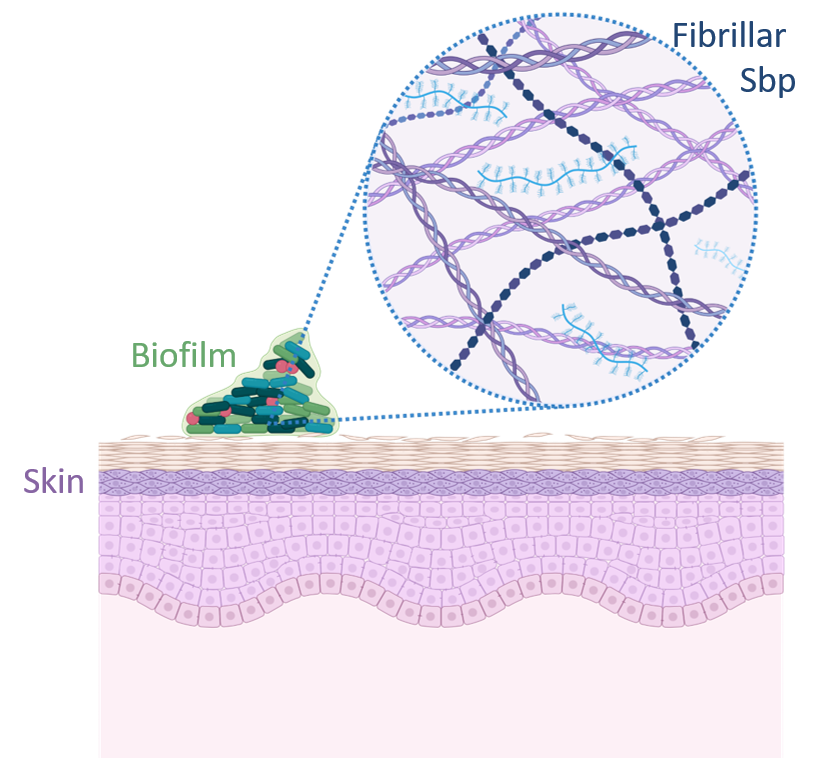
Nosocomial infections caused by Staphylococcus epidermidis are closely linked to the formation of biofilms and antibiotic resistance mechanisms, which include the presence of amyloid fibrils. Among these, some are constituted by a small basic protein (Sbp). Amyloids are a class of proteins secreted across various organisms, known for their ability to self-assemble into highly structured fibrils. These fibrils fulfill diverse physiological roles, impacting public health, environmental dynamics, and a range of industries and technologies significantly. Their pivotal role as major virulence factors in microbial pathogens makes them leading targets for structural analysis, with the goal of developing innovative antimicrobial treatments.However, our understanding of microbial amyloids lags behind especially in a physiological context. This gap is especially pronounced for SBP, where detailed insights into their structures and how they interact within biofilms are still elusive mostly due to the limits of imaging technologies. To address this knowledge gap, this project is dedicated to elucidating the structures of recombinant SBP using cryo-electron microscopy (cryo-EM). Furthermore, we aim to uncover the molecular functions of SBP within biofilms in their native context. This will be achieved through the application of cryo-electron tomography (cryo-ET) and correlative light and electron microscopy (cryo-CLEM). Combining these advanced imaging techniques with new image processing tools, we will gain unprecedented insights into the structural and functional dynamics of SBP within medically relevant biofilms, paving the way for novel antimicrobial strategies.




